 A major change in Rome III was the development of a diagnostic questionnaire which asked subjects to report on the frequency of occurrence of symptoms on an ordinal scale rather than responding only whether the symptom occurred at least 25% of the time on a binary Yes/No scale. The Rome Foundation board commissioned a multi-site study to develop and validate this questionnaire ($58,978).
A major change in Rome III was the development of a diagnostic questionnaire which asked subjects to report on the frequency of occurrence of symptoms on an ordinal scale rather than responding only whether the symptom occurred at least 25% of the time on a binary Yes/No scale. The Rome Foundation board commissioned a multi-site study to develop and validate this questionnaire ($58,978).
-
The goals of the project were to:
- compare the psychometric properties of different response scales in order to select the best one,
- assess the understandability of individual questions and revise them as needed,
- identify frequency thresholds for each symptom that are clinically meaningful and that separate FGID patients from healthy controls,
- assess the sensitivity and specificity of the diagnostic criteria and questionnaire, and
- assess the reliability of questionnaire-based diagnoses over a two-week interval.
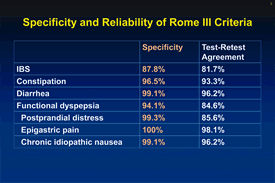
The sites participating in the project were the University of North Carolina (responsible for overall project management), Mayo Clinic, University of Toronto, and University of Michigan. Subjects for the study were 399 patients with clinically confirmed FGID diagnoses and 554 healthy controls. This project was completed in a 6-month period and project data were used to refine the diagnostic criteria and to finalize the diagnostic questionnaire (see Figure). The final report was published in the Rome III book, and the questionnaire and diagnostic scoring algorithm were made available on the web site,www.theromefoundation.org/questionnaires
